China conditionally approves four self-developed COVID-19 vaccines: official
Share - WeChat

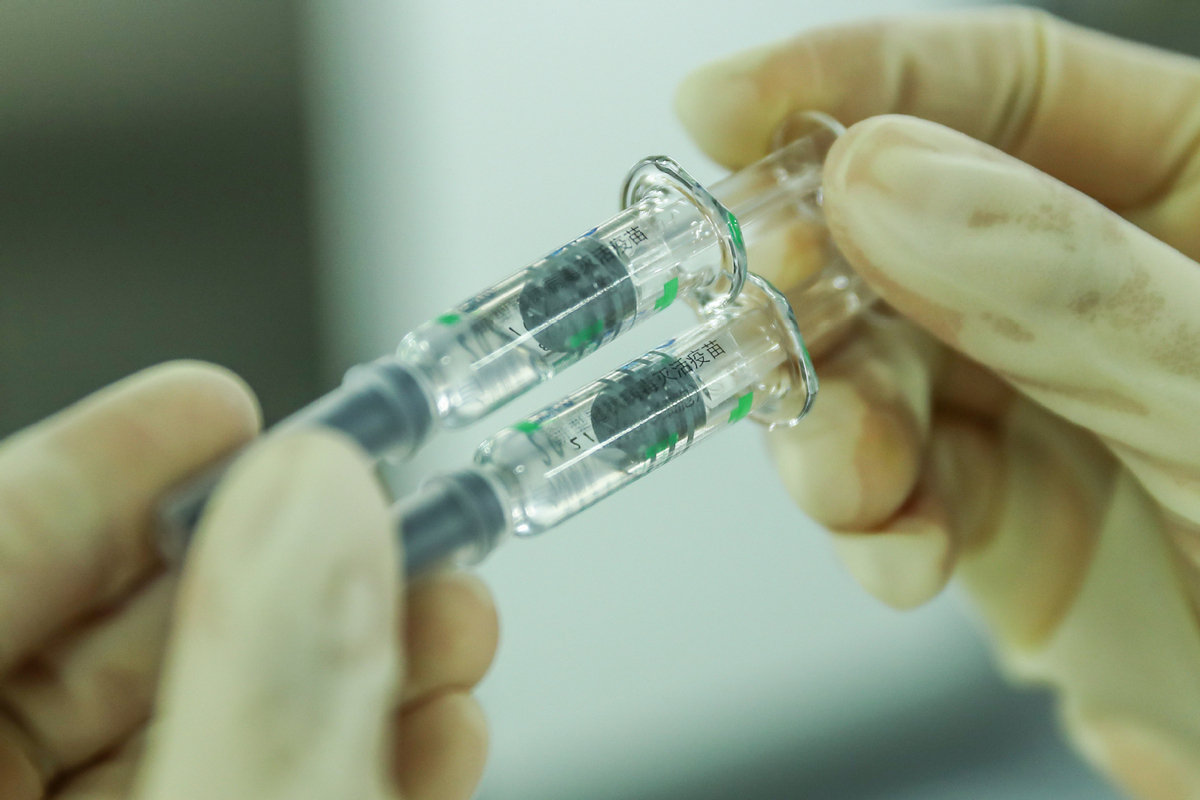
BEIJING - China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.
- Senior Xi'an official facing probe by China's anti-corruption watchdogs
- Philippines risks creating trouble for itself: China's defense ministry
- Newborn with congenital heart disease receives life-saving surgery in Yunnan
- Hong Kong charity signs diplomatic talent deal with Beijing university
- Aircraft carrier Fujian, commissioned
- Erdos offers 10,000 yuan subsidy for families having third child